Sonopet iQ
Ultrasonic Aspirator System
The Sonopet iQ Ultrasonic Aspirator not only delivers the power and control you need to fragment, emulsify and aspirate soft tissue and bone, but is also equipped with on-demand customizable features and RFID-enabled intelligence to help optimize your performance.
Contact us to request a trial and learn more about the clinical benefits.
CONTACT US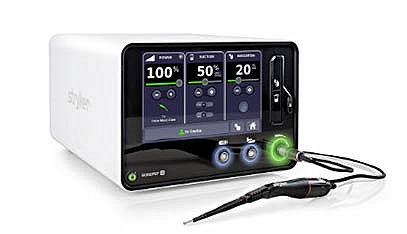
Simply brilliant.
Now with Pulse Control technology - gain an added ability to regulate the resection of hard and soft tissues using Sonopet iQ. Along with tip selection, power, suction and irrigation, Pulse Control gives you a new way to manage resecting complex tissue how you want.1
The Sonopet iQ system delivers even more of what our customers love. We’ve raised simplicity and reliability to an even higher level for O.R. surgical experiences. We’ve also added semi-autonomous functionality and customization so you can do more with the Sonopet iQ with less time and effort.2

A. Performance
- Handpiece refinements designed for power and reliability
- Designed for controlled suction pinch valve
- Greater irrigation control and auto prime ability
B. Customization
- RFID technology instantly changes settings based on connected tip or user profile
- One-step retrieval of surgeon profile
- Independent or synchronized settings for power and suction
C. Versatility and value
- One system – unparalleled customization
- One handpiece for soft tissue and bone cutting tips
- Enhanced guidance through large touchscreen interface
- Hand controller option for remote control from sterile or non-sterile field
D. Ease of Use
- One-click cassette and integrated back-end tubing connection
- On screen system setup and tutorials
- Alignment dots and color-changing ports that provide connection feedback
compared to Sonopet
compared to Sonopet
compared to Sonopet
Sonopet iQ's universal handpiece tip portfolio
Take control
Choose from basic, advanced and wireless foot pedals and a hand controller* for remote control from sterile or nonsterile field.


Wireless

Wired

Basic
Pulse Control:
Extrasensory ultrasonics

Suction and irrigation continue to flow during tip modulation when amplitude and energy are lower, allowing thermal heat from fragmentation to dissipate.1

Your singular bone cutting solution
The Sonopet iQ system fulfills diverse needs through a single handpiece and console. With integrated power, suction and irrigation, you'll have the precise tips and touch needed to gain access, cut, reshape, repair and realign.
With Sonopet iQ's universal handpiece, customization capabilities, semi-autonomous functionality and simple setup, the Sonopet iQ system aims to expand your bone cutting capabilities while streamlining capital costs, staff training, workspace and inventory.


Sonopet iQ setup video
Related products
Bone Mill+
Learn moreπdrive 2+
Learn moreSend us a message
- Stryker data on file. D0000115356 Rev. AA. S2200.211538. S2200.211539.
- All comparative metrics and claims are Sonopet iQ compared to Sonopet 1. Stryker internal test data on file. D0000004263; D0000012177 S2200.181435; S2200.180907
- Sonopet iQ has improved suction control for delicate resection rates with 75% lower achievable pressure settings compared to the first generation Sonopet. Stryker internal data. D0000012177 Rev AA.
- The Sonopet Ultrasonic Aspirator is currently being used in 104 out of 112 ACGME teaching institutions in the US. Stryker internal data on file. D0000002376. Temperature Reduction vs. Resection rate Engineering Notebook. D0000096235.
- Stryker data file. D0000129071 Rev. AA
- D0000248329
- D0000073709, Engineering Notebook 10cm iQ Broad Knife Low Gain Theory, AA.1.
NS-GSNPS-SYK-1369698_REV-0
Last Updated February/2025
























